mNSCLC BURDEN
Even with considerable progress
Survival rates remain low in mNSCLC1-5

Lung cancers, the majority of which are NSCLC, are the most commonly diagnosed cancers and the leading cause of cancer-related deaths globally6,7*

In the US, the majority of patients with lung and bronchus cancer are staged as metastatic at time of diagnosis8

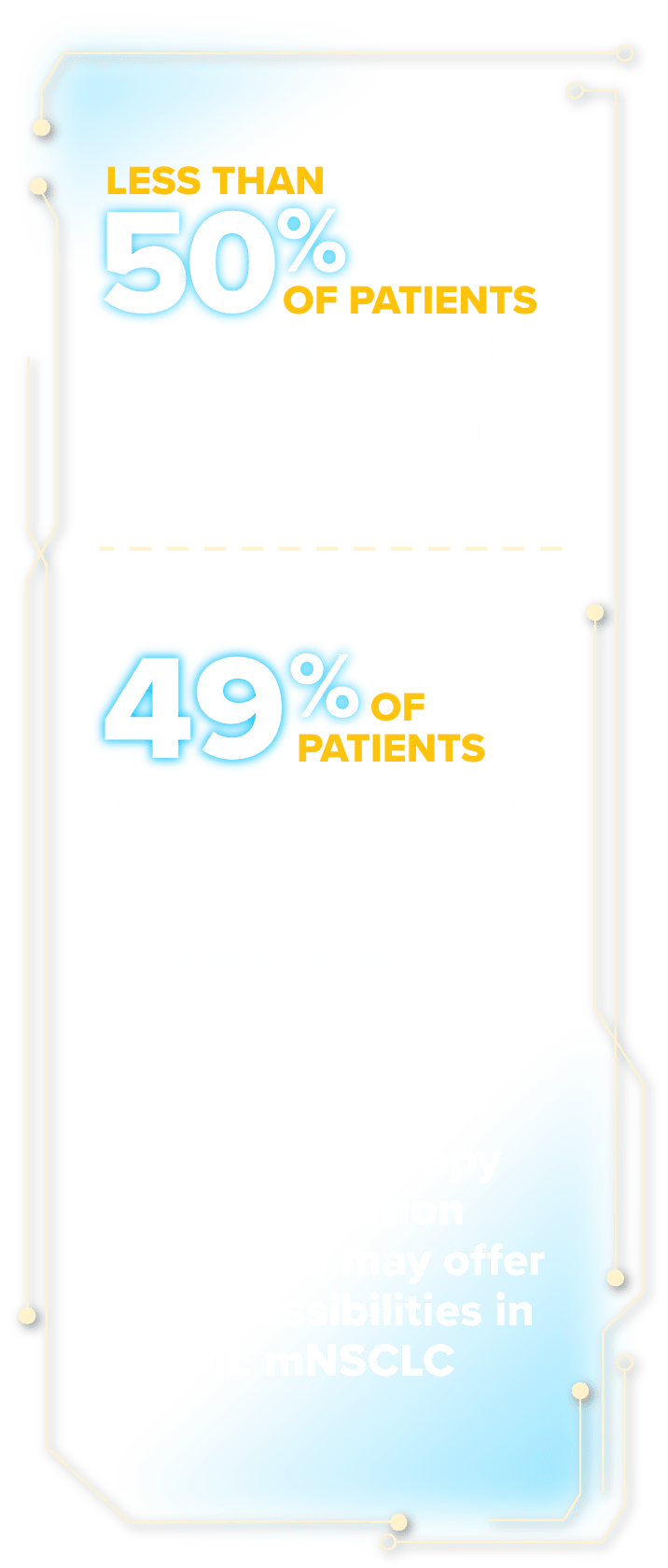
Current outcomes in mNSCLC with PD-L1 ≥50% show ~2 years median overall survival (OS)‡ and ~25% to ~30% 5-year survival
rate1-5§
*Data from GLOBOCAN 2022.7
†Based on data from the SEER Cancer Stat Facts: Lung and Bronchus Cancer derived from SEER 21 data for cases diagnosed between 2015-2021.8
‡As demonstrated in a pooled analysis of 12 randomized trials investigating anti–PD-(L)1 ± chemotherapy in 1L mNSCLC with PD-L1 ≥50%. Median OS was 25 months in the chemotherapy + anti–PD-(L)1 group (n=455) and 20.9 months in the anti–PD-(L)1 monotherapy group (n=1298).1
§Based on data from 4 randomized phase 3 studies investigating anti–PD-1 ± chemotherapy in 1L mNSCLC with PD-L1 ≥50% for patients without AGAs.2-5
FOR 1L mNSCLC
Many patients
currently experience
relapse or
are unable to achieve an initial
response
Multiple factors, including heterogeneity of clinical characteristics and tumor cells, contribute to mNSCLC treatment challenges, resulting in varied therapeutic responses.

MONTHS
median OS with current single-agent immunotherapy ± chemotherapy regimens in patients with PD-L1 ≥50%, based on results of clinical trials.2,4,9#
|| Based on ORR from 4 randomized phase 3 studies of 1L mNSCLC in patients without AGAs treated with a PD-1 or PD-L1 inhibitor.9-12
¶ As seen in 243 patients with mNSCLC who initially responded to anti–PD-1 treatment at Memorial Sloan Kettering Cancer Center from April 2011 to December 2017. Acquired resistance was defined as partial or complete response followed by isolated or systemic progression on or before the date of last scan.13
#Based on the 5-year follow-ups of 3 randomized phase 3 studies of 1L mNSCLC in patients without AGAs treated with a PD-1 inhibitor ± chemotherapy.2,4,9

REAL-WORLD EVIDENCE
OS for
1L mNSCLC in
PD-L1 ≥50%14
Data from a noninterventional, observational study conducted in patients with a confirmed diagnosis of advanced NSCLC and no AGAs (identified from the Flatiron Health database; United States) between January 2011 and June 2020. Patients received either 1L single-agent immunotherapy (N=3041) or 1L immunotherapy + chemotherapy (N=4271) on or after January 1, 2016. The primary study objective was to evaluate real-world OS with immunotherapy ± chemotherapy.


Study limitations:
- Retrospective and observational design, limited follow-up, and inconsistency of some clinical information collected
- No formal hypothesis testing was performed; analyses are not intended to be compared to controlled clinical trial data
EMERGING POTENTIAL
EMERGING POTENTIAL
Powering possibilities in mNSCLC with TROP-215
TROP-2, a proven target in multiple cancers, is a calcium signal transducer involved in several signaling pathways related to:
- Cell proliferation through cyclins and cyclin-dependent kinases
- Invasion and metastasis via matrix metalloproteinases and other proteins
- Cell survival by degradation of FOXO3a
TROP-2 is expressed in 89% to 100% of NSCLC, regardless of tumor histology or presence of actionable alterations.16-18
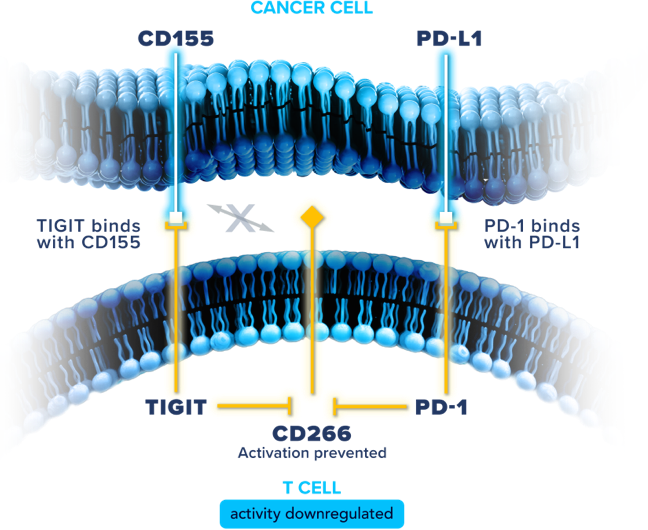
Illuminating potential in mNSCLC through the TIGIT and PD-1 pathways19-21
TIGIT and PD-1 are
among the immune checkpoints often distinctly
co-expressed on activated immune cells. While PD-1 pathway activation
and its associated downstream effects on immune response have been well
studied in mNSCLC, there may be opportunities for further therapeutic
impact. Preclinical data indicate that combined inhibition of the TIGIT
and PD-1 pathways may improve
T cell proliferation and increase cytokine
secretion to enhance T cell activity, potentially addressing the body’s
downregulated antitumor response.
STAY INFORMED
1L=first-line; AGA=actionable genomic alteration; mNSCLC=metastatic non-small cell lung cancer; mOS=median overall survival; NSCLC=non-small cell lung cancer; OS=overall survival; PD-1=programmed cell death protein 1; PD-L1=programmed cell death protein ligand 1; QOL=quality of life; TIGIT=T cell immunoreceptor with immunoglobulin and ITIM domain; TROP-2=trophoblast cell-surface antigen 2.
References:
- Akinboro O, Vallejo JJ, Nakajima EC, et al. Outcomes of anti–PD-(L)1 therapy with or without chemotherapy (chemo) for first-line (1L) treatment of advanced non–small cell lung cancer (NSCLC) with PD-L1 score ≥50%: FDA pooled analysis. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 2022; Chicago, IL.
- Garassino MC, Gadgeel S, Speranza G, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non–small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41(11):1992-1998.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥50%. J Clin Oncol. 2021;39(21):2339-2349.
- Novello S, Kowalski DM, Luft A, et al. Pembrolizumab plus chemotherapy in squamous non–small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. 2023;41(11):1999-2006.
- Kilickap S, Baramidze, A, Sezer A, et al. Cemiplimab monotherapy for first-line treatment of patients with advanced NSCLC with PD-L1 expression of 50% or higher: five-year outcomes of EMPOWER-Lung 1. J Thorac Oncol. 2025;S1556-0864(25)00178-9. doi: 10.1016/j.jtho.2025.03.033
- Schabath B, Cote ML. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1563-1579.
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263.
- National Cancer Institute: SEER 21. Cancer Stat Facts: Lung and Bronchus Cancer. Accessed May 16, 2025. https://seer.cancer.gov/statfacts/html/lungb.html
- de Castro Jr G, Kudaba I, Wu Y-L, et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥1% in the KEYNOTE-042 study. J Clin Oncol. 2023;41(11):1986-1991.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
- Jassem J, de Marinis F, Giaccone G, et al. Updated overall survival analysis from IMpower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol. 2021;16(11):1872-1882.
- Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592-604.
- Memon D, Schoenfeld AJ, Ye D, et al. Clinical and molecular features of acquired resistance to immunotherapy in non-small cell lung cancer. Cancer Cell. 2024;42(2):209-224.
- Waterhouse D, Lam J, Betts KA, et al. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer. 2021;156:41-49.
- Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015;6(3-4):84-105.
- Omori S, Muramatsu K, Kawata T, et al. Trophoblast cell-surface antigen 2 expression in lung cancer patients and the effects of anti-cancer treatments. J Cancer Res Clin Oncol. 2022;148(9):2455-2463.
- Mito R, Matsubara E, Komohara Y, et al. Clinical impact of TROP2 in non-small lung cancers and its correlation with abnormal p53 nuclear accumulation. Pathol Int. 2020;70(5):287-294.
- Kuo P, Elboudwarej E, Diehl L, Patel J, Mekan S, Jürgensmeier JM. Trop-2 expression in non-small cell lung carcinoma. Poster presented at: American Association for Cancer Research Annual Meeting; April 14-19, 2023; Orlando, FL.
- Banta KL, Xu X, Chitre AS, et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity. 2022;55(3):512-526.
- Lin X, Kang K, Chen P, et al. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol Cancer. 2024;23(1):108. doi: 10.1186/s12943-024-02023-w
- Nutsch K, Banta KL, Wu TD, et al. TIGIT and PD-L1 co-blockade promotes clonal expansion of multipotent, non-exhausted antitumor T cells by facilitating co-stimulation [published correction appears in Nat Cancer. 2025 Feb;6(2):405. doi: 10.1038/s43018-025-00908-3.]. Nat Cancer. 2024;5(12):1834-1851.